Take Action | Animal Tests | Q&A: Chemicals
-

Rats squeezed into full-body restraint tubes during an inhalation toxicity test. NIEHS
-
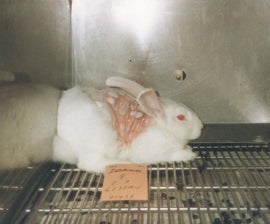
Rabbit “lethal dose” skin tests cause needless suffering. Photobucket
-

Reproductive and developmental toxicity studies consume between 1,300 and 2,600 mother and baby rats per chemical tested. Photobucket
An estimated 100,000 chemicals are marketed globally, with hundreds more new chemicals being introduced each year. Most are plastics and related polymers, while a smaller proportion include cleansers, paints, adhesives, lubricants, industrial solvents and a variety of short-lived by-products or “intermediates.” Some are kept tightly contained in closed systems and never released into the environment, while others may be marketed in high volumes and/or used as ingredients in products to which human beings and the environment may be exposed (e.g., cosmetics and household cleaning products, plastic packaging, and gasoline. Recently implemented laws in Europe, China and elsewhere are requiring companies to produce large quantities of test data, which could mean suffering and death for tens of millions of animals.
Within REACH
Europe’s Registration, Evaluation and Authorisation of Chemicals (REACH) regulation of 2007 imposes escalating information (testing) requirements according to the volume in which a chemical is manufactured or imported each year. Estimates of potential new animal testing under REACH range from 9 million to as many as 45 million.
At the same time, REACH includes a number of strict measures aimed at avoiding new animal testing. One of these directs the European Commission, “as soon as possible” after a suitable animal testing alternative becomes available, to initiate proceedings to amend REACH testing requirements “so as to replace, reduce or refine animal testing.” Yet despite the availability of many such new and validated alternative test methods since 2007, no action has yet been taken to revise REACH testing requirements.
HSI has submitted a comprehensive proposal to the European Commission calling for major revisions to REACH data requirements to reflect scientific progress and minimize new animal testing to the maximum possible extent. TAKE ACTION »
Donate to support HSI’s campaign to end animal testing around the world.
