-
AHWLA
The European Pharmacopoeia Commission and the United States Department of Agriculture have recently revised their requirements for the safety assessment of vaccines to remove or waive two controversial and scientifically unnecessary animal tests, a move that could spare scores of animals going forward. Humane Society International and The Humane Society of the United States welcome these developments, and call on vaccine manufactures to be pro-active in seizing these opportunities to end the use of animal testing on their products, and on other national vaccine authorities to follow these positive examples.
In Europe, the “Abnormal Toxicity Test” used in batch safety testing of human vaccines will be removed from all pharmacopoeia monographs effective 2019, and in the U.S., authorities recently announced that they will now permit replacement of the Target Animal Batch Safety Test for veterinary vaccines with non-animal quality controls and production consistency. These vaccine batches are tested on untold thousands of animals every year, given their repeated use to test every new batch of a vaccine. Globally, vaccine batch-release testing is believed to account for up to 15 percent of the more than 100 million animals used each year in laboratory experiments.
Troy Seidle, HSI senior director for research & toxicology, said: “We recognize the years of investment by many corporate and governmental stakeholders that have brought animal testing alternatives for vaccines to this point, and are committed to supporting and expanding this humane revolution on a global basis. We believe the extremely animal intensive field of vaccine testing is ripe for change through facilitated stakeholder dialogue, cooperation and science-based regulatory reform.”
Facts:
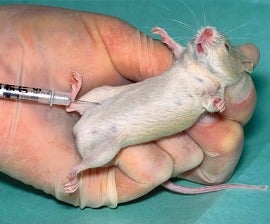
- The Abnormal Toxicity Test dates back to the early 1900s, and involves injecting a defined amount of vaccine from a production batch into mice or guinea pigs, followed by a period of clinical observation. Its removal from the European Pharmacopoeia is a product of years of dialogue supported by the European Partnership for Alternative Approaches to Animal Testing, with similar proposals having been made by the U.S. Food and Drug Administration and several other national authorities. However, the test is still required by vaccine authorities in Argentina, China, Japan, Mexico and other countries.
- The Target Animal Batch Safety Test is an overdose test using either the target species (mammals, birds, fish, etc.), or mice and guinea pigs, performed on each batch of vaccine to assess its safety. Animals are injected with two- to 10-times the human dose of a vaccine and observed for signs of illness or death. The test was almost fully deleted from the European Pharmacopeia in 2012, but is still required in other major markets, including Brazil, China, India and South Korea.
Media contact: please fill out our media relations contact form here.
